Brain tumour cells rapidly integrate into brain-wide neuronal circuits
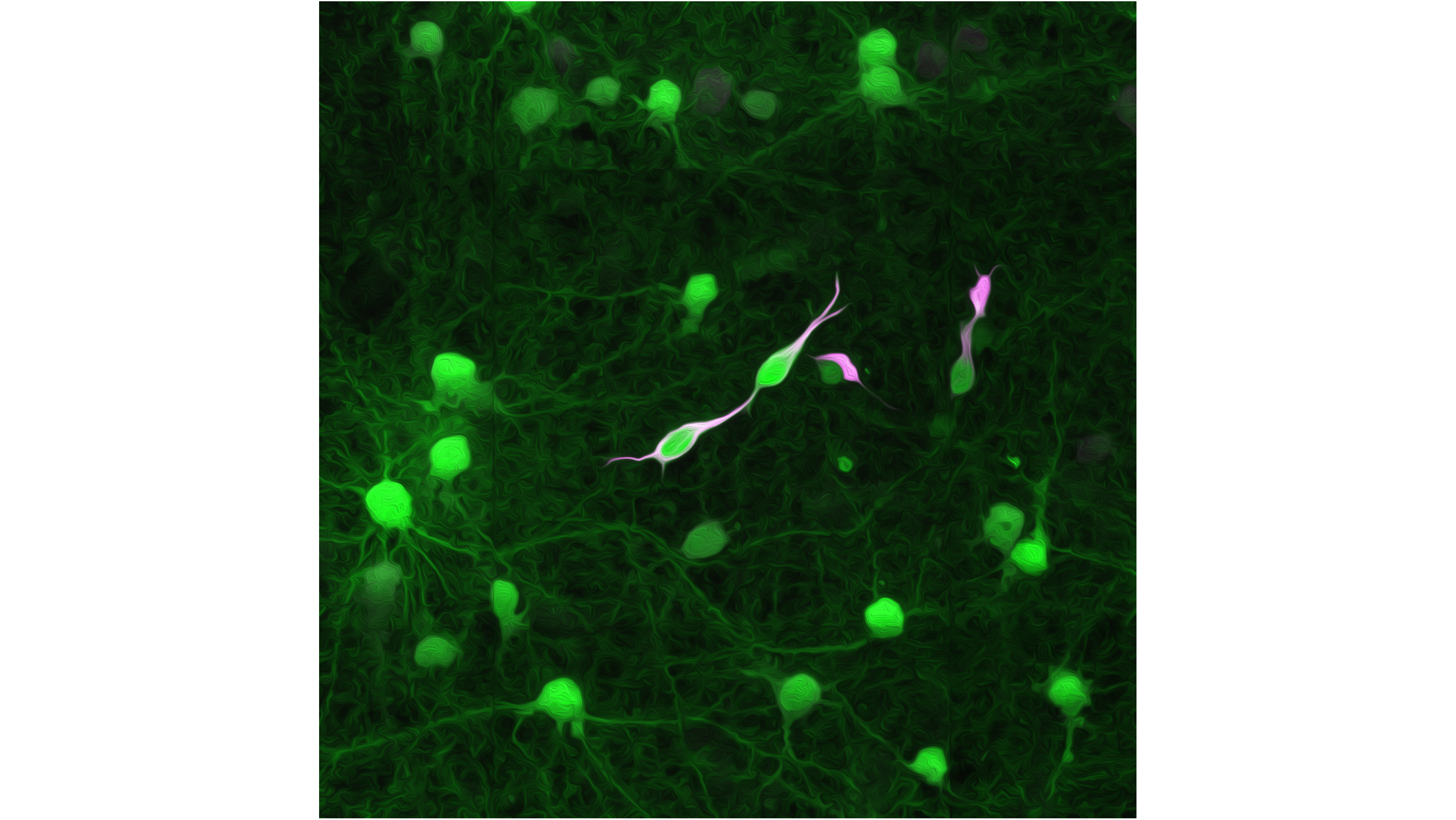
Researchers at the Medical Faculty of Heidelberg University and the Heidelberg University Hospital have used modified rabies viruses to label glioblastoma tumour cells and their direct cell contacts in the mouse brain. The new method showed that the tumour cells are connected to different types of nerve cells throughout the entire brain at a very early stage of the disease. This means that they form a network of connections with brain cells much earlier than previously assumed. It is this network that makes these tumours so difficult to treat. The results have been published in the latest edition of the scientific journal ‘Cell’.
The tumour cells of highly aggressive glioblastomas grow into the brain like a mycelium. This invasion is promoted by the nerve cells of the brain itself as they form cell-cell contacts with the tumour cells and pass on excitatory signals to them. Using a new method, researchers from Heidelberg University, the University Hospital (UKHD) and the German Cancer Research Center (DKFZ) have now shown that this contact occurs much earlier and also involves more types of nerve cells than previously assumed. The team, led by Dr. Dr. Varun Venkataramani, a neurologist and research group leader at the UKHD, infected and labelled human glioblastoma cells with modified rabies viruses and tracked how contact-seeking nerve cells became infected in human tissue models and in the mouse brain. The researchers hope to use their new findings for future therapies for glioblastomas, which are currently incurable.
‘The tumour network makes glioblastomas so difficult to fight: they cannot be completely removed and their interconnection makes them almost insensitive to radiation and chemotherapy,’ says Professor Dr. Wolfgang Wick, Medical Director of the UKHD’s Department of Neurology, Head of the European Centre for Neuro-Oncology at the Medical Faculty of Heidelberg University and Head of the ‘Neuro-Oncology’ Clinical Cooperation Unit of the UKHD and DKFZ. He is the spokesperson for the UNITE GLIOBLASTOMA Collaborative Research Centre, which is coordinated from Heidelberg and in which the recently published work was carried out. ‘Every new insight into how these tumours “tick” and where their weak points might be is a valuable step in the development of future therapies.’ Despite modern therapeutic strategies, patients diagnosed with glioblastoma have a less than two-year average survival rate.
Rabies virus spreads from infected tumour cells to contact-seeking nerve cells
Dr. Dr. Venkataramani’s idea was to use a virus that specialises in infecting the nervous system to combat the tumour cells: rabies viruses are usually transmitted via the bite of an infected animal and attack nerve cells. From the bite wound, they migrate along the neural connections into the brain, where they cause life-threatening inflammation. ‘We have exploited the ability of rabies viruses to pass from one nerve cell to the next via their contact points,’ says the neurologist.
Changes to the virus genome ensure that the viruses only pass from the tumour cell to directly connected nerve cells. Transmission from these to other nerve cells is not possible. In addition, the modified virus transfers the genetic blueprint for proteins that are fluorescent, thus making both tumour cells and their direct contact partners visible. ‘With previous techniques, these cell-cell contacts could only be traced in the immediate vicinity of the tumour. With the help of the rabies viruses, we can now also see the contact partners that connect with the tumour cells over long distances via long cell extensions,’ says Svenja Tetzlaff from Dr. Dr. Venkataramani’s team, one of the two lead authors of the article. ’We can now map the entire network of tumour-nerve connections in the brain.’
Aggressively invasive growth even in the very early stages of the disease
Molecular contact tracing showed that the tumour cells connect to nerve cells extremely quickly. Long before the tumour becomes visible using clinical imaging and long before neurological disorders occur, the cancer cells are already linked to neuronal networks. ‘We did not expect this. It means that the aggressive growth of these brain tumours occurs at a very early stage, long before the first signs of disease,’ says Ekin Reyhan, a junior scientist at the Heidelberg Medical Faculty and also a first author on the paper.
In addition, the new method of visualising more distant contact partners revealed several different types of nerve cell for the first time. However, so-called acetylcholinergic nerve cells, which are important for memory and attention processes, appear to play a special role. In animal experiments, tumour growth slowed down when the tumour cells were genetically modified so that they could no longer receive signals from these nerve cells.
Combination of radiation and targeted drugs could improve therapy
The close contacts with healthy nerve cells also help the tumour network to survive radiation better, even if the main tumour in the core radiation area dies, as the scientists discovered: in mice, the radiation increased neuronal activity, so more excitatory signals reached the remaining tumour cells and thus fuelled their spread. When the researchers dampened the overactivity of the nerve cells with a specific epilepsy drug, the radiotherapy had a more lasting effect, and the glioblastoma regenerated much more slowly. ‘This could be highly relevant for therapy,’ says Dr. Dr. Venkataramani. ‘However, since the results of animal experiments cannot be transferred 1:1 to humans, we will only know for sure after clinical trials with patients.’ The modified rabies viruses could also be used to block the nerve cells in contact with the tumour. The team showed that this is possible in principle. However, extensive modifications to the virus are still needed before it can be used in humans.
Literatur
Tetzlaff SK, Reyhan E, Layer N, et al. Characterizing and targeting glioblastoma neuron-tumor networks with retrograde tracing. Cell. Published online December 6, 2024. doi:10.1016/j.cell.2024.11.002
Weitere Informationen im Internet
Klinische Kooperationseinheit Neuroonkologie
Kontakt
Dr. med. Dr. rer. nat. Varun Venkataramani
Research group leader and resident physician in neurology
Neurological Clinic of the UKHD
Department of Functional Neuroanatomy
Institute of Anatomy and Cell Biology
Heidelberg Medical Faculty of the Heidelberg University
Clinical Cooperation Unit Neuro-Oncology, German Cancer Research Center
E-mail: varun.venkataramani@med.uni-heidelberg.de




