
Network News

Ladenburg, Germany, 23 October 2025 – Heidelberg Pharma AG (FSE: HPHA), a clinical-stage biotech company developing innovative Antibody Drug Conjugates (ADCs), today announced that HDP-101 (INN: pamlectabart tismanitin), the Company’s lead Amanitin-based ADC candidate, has been granted Fast Track Designation by the US Food and Drug Administration (FDA). This designation was supported by nonclinical data as […]
read more
GalaTek GmbH and a:head bio AG Announce Strategic Collaboration to Automate Human Brain Organoid Workflows
Vienna, Austria – October 22, 2025 – GalaTek GmbH, a leading innovator in laboratory automation technologies, and a:head bio AG, a pioneering biotech company specializing in human brain organoid research, today announced a strategic collaboration aimed at advancing the automation of brain organoid workflows. The partnership aims to integrate GalaTek’s advanced robotics and software platforms with […]
read more
Clean Tech Innovation Day & Solar TAP Industry Day: Advanced solutions are needed
Heidelberg, Germany, October 22, 2025 – It’s all about a sustainable, smarter energy future: Clean Tech Innovation Day & Solar TAP Industry Day showed that this can only be achieved with a shared mission and vision. More than 70 participants from science, industry, and start-ups took the opportunity at the Heidelberg Congress Center to exchange […]
read more
Sanofi’s Wayrilz recommended for EU approval by the CHMP to treat immune thrombocytopenia
Sanofi’s Wayrilz recommended for EU approval by the CHMP to treat immune thrombocytopenia Paris, October 17, 2025. The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion recommending the approval of Wayrilz (rilzabrutinib) as a new treatment for immune thrombocytopenia (ITP) in adult patients who are refractory to other […]
read more
NGS-based diagnostics for identifying sepsis pathogens wins EARTO Innovation Award
A method developed at the Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB enables bacterial, viral, fungal, or parasitic pathogens in sepsis patients to be identified much faster than before and with the highest precision. The approach is based on high-throughput sequencing of cell-free DNA circulating in the blood and was honored with the EARTO […]
read more
SPT Labtech and Agilent Introduce Automated Target Enrichment Protocols for Genomic Workflows
Cambridge, UK, 08 October 2025: SPT Labtech, a pioneer in the design and development of laboratory automation and liquid handling solutions, and Agilent Technologies Inc. (“Agilent”), a global leader in analytical and clinical laboratory technologies, today announced the introduction of automated target enrichment protocols on SPT Labtech’s firefly®+ platform. Automating genomic workflows, the optimized Target […]
read more
PHOENIX fördert Forschung: Pharmazie Wissenschaftspreis für Pharmazeutische Technologie verliehen
Herzlichen Glückwunsch an JunProf. Dr. Philipp Uhl von der Universität Heidelberg und sein Team. Für PHOENIX ist das eine ganz besondere Auszeichnung, denn als approbierter Apotheker mit Schwerpunkt in der pharmazeutischen Forschung verbindet Philipp Uhl Praxis und Forschung – genau das, was die pharmazeutische Versorgung von morgen braucht. Die Forschung des Teams könnte die Arzneimitteltherapie verändern: […]
read more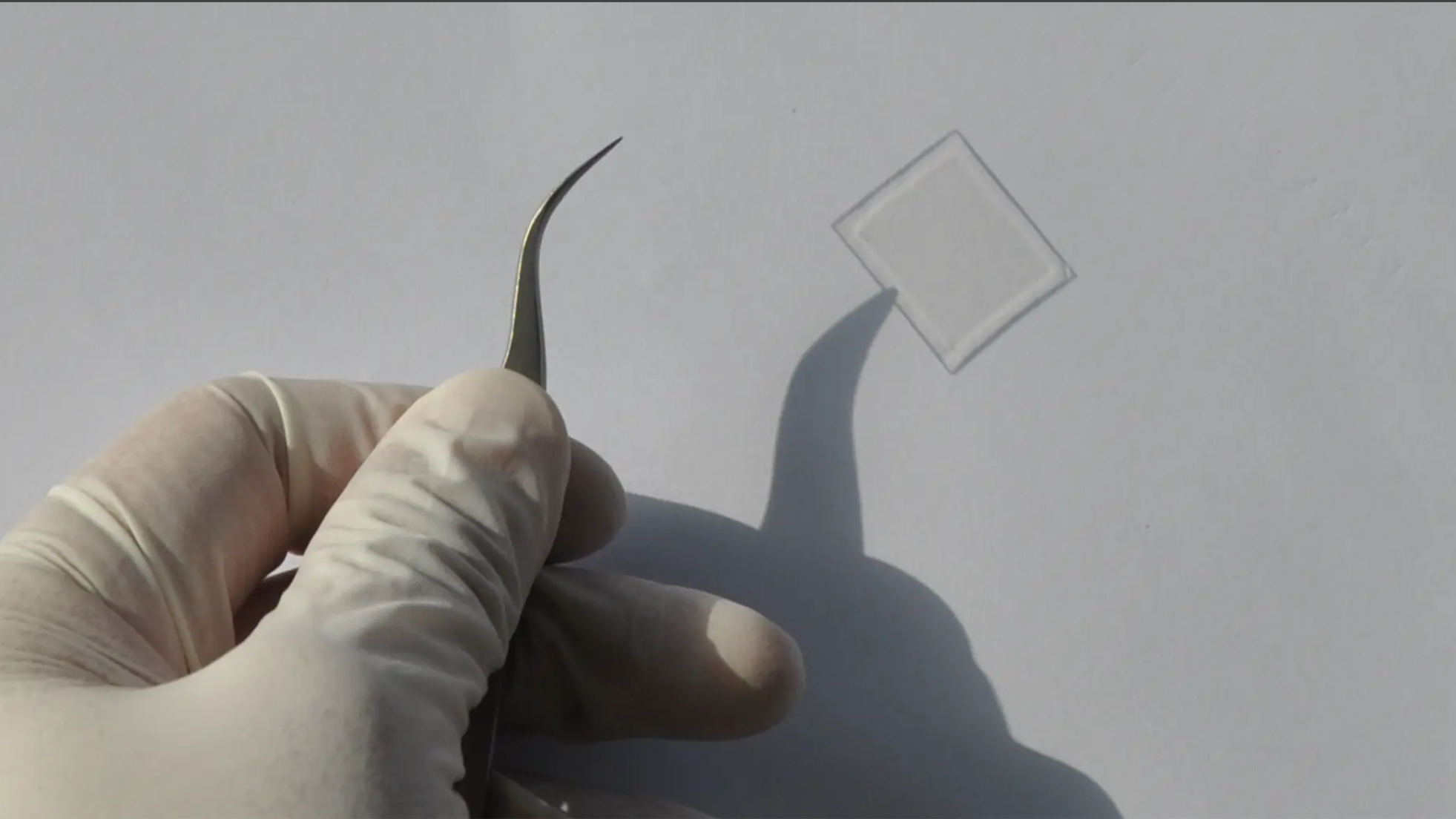
No more problems with reflective surfaces
Start-up nanoAR receives funding from a federal “exist”-Forschungstransfer grant for its anti-reflective surface technology Most people have been irritated by the reflection of sunlight on their smartphone display or their spectacles. Many solutions are available — but until now none for curved surfaces or situations with a wide range of light incidence angles. Scientists at […]
read more
WMT AG receives financing with participation from L-Bank’s “InnoGrowth BW” program
Marathon Beteiligungs AG is the lead investor, CARMA Funds also participates in €3.67 million round Heidelberg-based WMT AG today announced the completion of an equity financing round totaling €3.63 million. The lead investor, which was able to significantly expand its investment with the InnoGrowth BW program of L-Bank, is Dresden-based Marathon Beteiligungs AG, which is […]
read more
