Hepatic stellate cells control liver function and regeneration
Until now, doctors knew hepatic stellate cells mainly as drivers of liver fibrosis. The actual functions of this cell type have hardly been studied to date. Researchers from the German Cancer Research Center (DKFZ), the Mannheim Medical Faculty and Columbia University in New York have now published in the journal Nature that hepatic stellate cells control liver metabolism as well as liver regeneration and size. The results of the study could contribute to new therapeutic approaches for liver diseases.
The liver is a central organ for carbohydrate and protein metabolism as well as for the detoxification of endogenous and exogenous substances. Its vital functions require a high regenerative capacity. Liver cells, so-called hepatocytes, make up about 60 percent of the total cell number of the organ. They have important metabolic tasks and are active in detoxification and metabolism. Endothelial cells provide mass transfer, cholangiocytes transport bile, and Kupffer cells act as a protective barrier.
Hepatic stellate cells are located along the specialized blood capillaries of the liver, known as sinusoids. They are known to drive liver fibrosis, but their actual physiological functions and interactions with other cells are largely uninvestigated.
Scientists led by Hellmut Augustin, DKFZ and the Mannheim Medical Faculty of Heidelberg University, and Robert F. Schwabe, Columbia University, New York, have now shown that stellate cells are essential for liver regeneration and liver metabolism.
Using a genetic trick, the teams led by the two senior authors bred mice whose livers contained no stellate cells. This affected the organ’s detoxification function and its ability to regenerate after injury. The finely balanced architecture of the liver lobules, in which the hepatocytes are arranged according to their different metabolic tasks, was completely disrupted without stellate cells.
The protein Rspondin 3 (RSPO3), which is preferentially produced in stellate cells and regulates the important WNT signaling pathway in liver cells, proved to be a key player in this process. When the researchers specifically switched off RSPO3 in stellate cells, this genetic manipulation had the same effects as the loss of stellate cells.
“The results show that hepatic stellate cells are not only involved in pathological processes, but also play an active role in protecting and regulating liver function,” explains Hellmut Augustin. ”It is particularly noteworthy that the selective silencing of Rspondin 3 leads to similar effects as the removal of the stellate cells themselves – with serious consequences for liver health.”
The researchers also showed that low RSPO3 levels are associated with unfavorable disease progression in patients with alcohol-associated and metabolic liver disease. The new results provide a potential approach for future therapeutic strategies. Instead of focusing solely on inhibiting stellate cells to prevent the development of fibrosis, future treatments could specifically maintain and support their positive, protective functions.
Publication:
Atsushi Sugimoto, Yoshinobu Saito, Guanxiong Wang, Qiuyan Sun,,Chuan Yin, Ki Hong Lee, Yana Geng, Presha Rajbhandari, Celine Hernandez, Marcella Steffani, Jingran Qie, Thomas Savage, Dhruv M. Goyal, Kevin C. Ray, Taruna V. Neelakantan, Deqi Yin, Johannes Melms, Brandon M. Lehrich, Tyler M. Yasaka, Silvia Liu, Michael Oertel, Tian Lan, Adrien Guillot, Moritz Peiseler, Aveline Filliol, Hiroaki Kanzaki, Naoto Fujiwara, Samhita Ravi, Benjamin Izar, Mario Brosch, Jochen Hampe, Helen Remotti, Josepmaria Argemi, Zhaoli Sun, Timothy J. Kendall Yujin Hoshida, Frank Tacke, Jonathan A. Fallowfield, Storm K. Blockley-Powell,Rebecca A. Haeusler, Jonathan B. Steinman, Utpal B. Pajvani, Satdarshan P. Monga,Ramon Bataller, Mojgan Masoodi, Nicholas Arpaia, Youngmin A. Lee, Brent R. Stockwell, Hellmut G. Augustin & Robert F. Schwabe: Hepatic stellate cells control liver zonation, size and functions via Rspondin 3.
Nature 2025, DOI: https://www.nature.com/articles/s41586-025-08677-w
A picture is available for download:
PM_Augustin_Nature.jpg
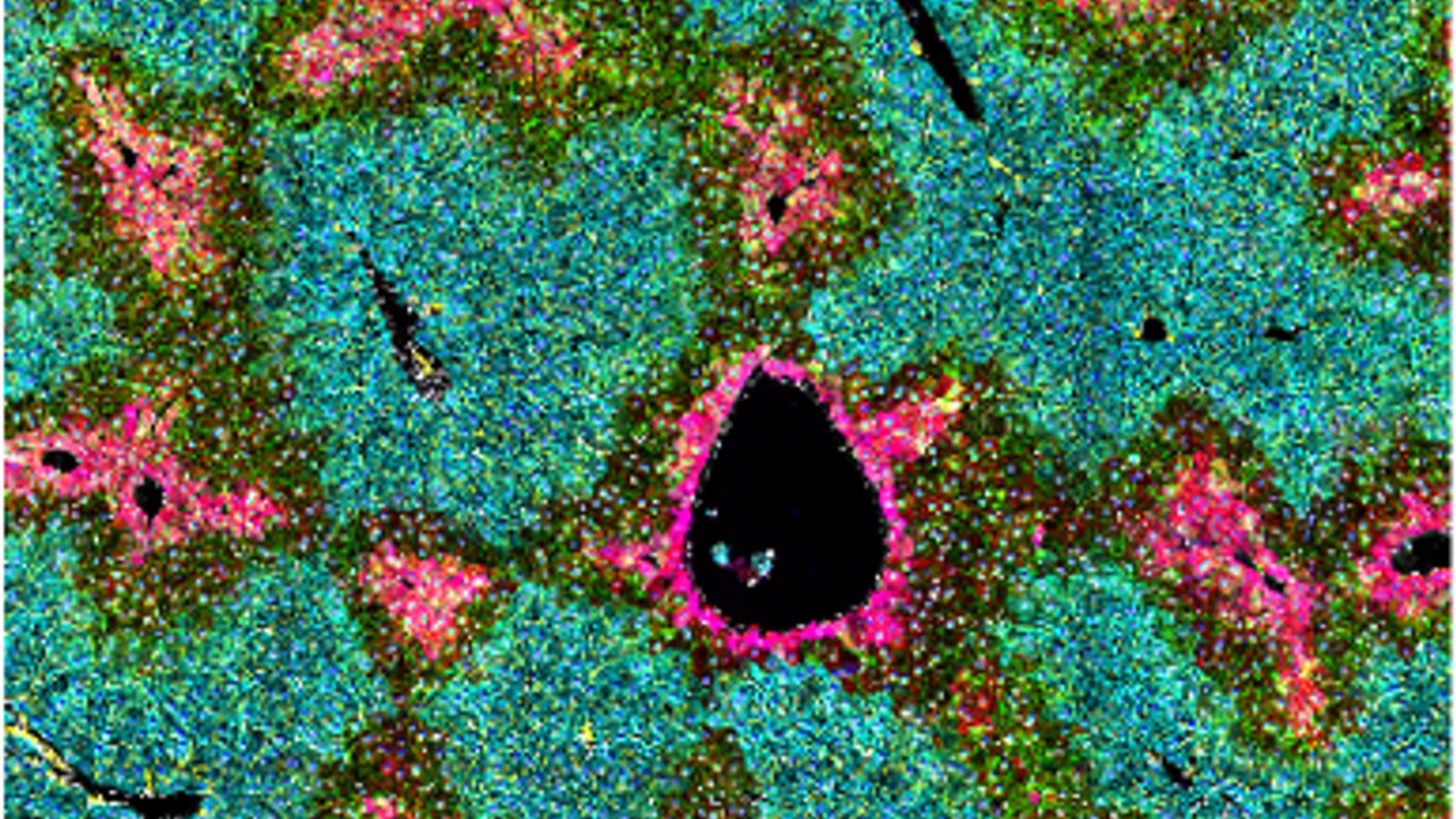
Caption: Hepatic stellate cells control the function of the liver. The different colors show specialized functions of the liver cells within the pentagonal to octagonal liver lobules.
Note on use of images related to press releases
Use is free of charge. The German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) permits one-time use in the context of reporting about the topic covered in the press release. Images have to be cited as follows: “Source: Augustin/DKFZ”.
Distribution of images to third parties is not permitted unless prior consent has been obtained from DKFZ’s Press Office (phone: ++49-(0)6221 42 2854, E-mail). Any commercial use is prohibited.




