NMI expertise for new VDI guideline on bioprinting – now available
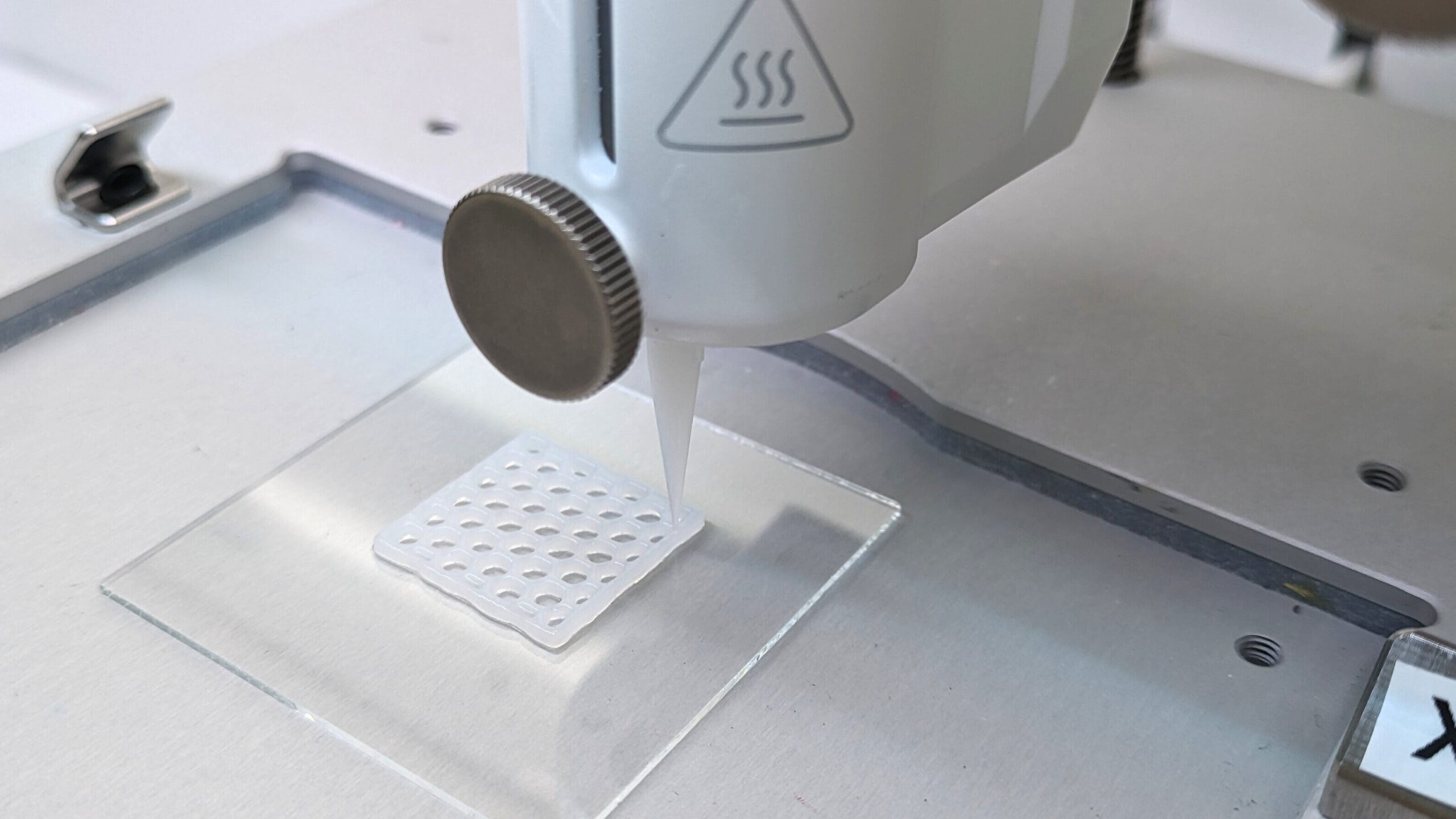
VDI Guideline 5708 “Bioprinting, methods and definitions”: What sounds technical and sober at first glance is an important step forward in the future field of 3D bioprinting. The guideline was developed under the leadership of Dr. Hanna Hartmann from the NMI Natural and Medical Sciences Institute in Reutlingen as chairwoman and Prof. Dr. Jürgen Groll from the University Hospital of Würzburg as deputy chairman. After around one and a half years of intensive committee work in the transdisciplinary committee, it was adopted as a white paper in April 2024. For the first time, it creates a binding, practical basis for reproducible and quality-assured bioprinting procedures.
Why a guideline? Uniformity, quality, comprehensibility
3D bioprinting is developing dynamically, but a lack of standards has so far made it difficult to compare results, control the quality of bioinks and reproducibly produce structures that can be used in regenerative medicine, to provide test systems for the pharmaceutical industry and for basic cell biology studies. The VDI guideline now closes this gap: It defines key terms, describes methodological principles, recommends minimum requirements for materials, processes and reproducibility and thus supports both research and industrial product development. It is explicitly aimed at all players along the value chain – from research institutions and manufacturers to users and testing laboratories.
Standardized terminology in a transdisciplinary field
With the new publication, the guideline provides manufacturers, researchers and users with a common language for the definition and application of 3D bioprinting technologies. “The transdisciplinary set-up of the guideline committee was a key concern for us,” says Dr. Hanna Hartmann, Head of Biomedicine and Materials Science at the NMI. “It is a decisive step towards harmonizing research and development.” The technical rule has been available via DIN-Media since May 1st: https://www.dinmedia.de/de/technische-regel/vdi-5708-blatt-1/384316908.
Interdisciplinary committee with a clear practical orientation
Under the chairmanship of Dr. Hanna Hartmann and Prof. Dr. Jürgen Groll, an interdisciplinary committee with representatives from research, industry, application and certification worked on the new guideline. In ten meetings, key terms were defined, requirements for materials, processes and quality assurance were formulated and the results were prepared in a practical manner. “VDI 5708 is an important step towards comprehensible and comparable standards,” explains Prof. Jürgen Groll. “These are essential for the development of future products – whether in regenerative medicine, drug testing or in-vitro diagnostics.”
SOP_BioPrint as a basis – funded by the BMBF
The technical basis for the guideline comes from the SOP_BioPrint project (FKZ: 13XP5071C) funded by the Federal Ministry of Education and Research (BMBF). The aim was to develop standardized processes for bioprinting – which have now been incorporated into the guideline.
Further information on the SOP_BioPrint project can be found on the NMI project page. The BMBF brochure also provides an overview of current developments in bioprinting: Bioprinting – Potenziale für medizinische Forschung und Anwendung.




