Novel NKG2D-targeting bispecific antibodies improve B celllymphoma killing

Combination approach could potentiate the success of immunotherapy
MEDICAL NEED: Owing to advances in cancer research, mortality rates have decreased in recent years.1
However, there are many patients who do not benefit from the plethora of available therapies because
tumors develop diverse immune escape mechanisms. Therefore, further development, investigation and
improvement of current and new therapies is imperative.
Therapeutic monoclonal antibodies (mAb) are a cornerstone of immunotherapy with recruitment and stimulation of effector cells as a prominent goal. As a versatile tool, they are used as naked antibody, in antibody-drug conjugates to deliver toxic agents, as multi-specific constructs to simultaneously target different epitopes, as antibody-cytokine fusion proteins to induce cytokine production, and many more.2 However, this process is hindered in some patients.
Strategies to improve effector cell engagement include Fc-engineering, combination of different mAb or the development of further bispecific antibodies (bsAbs) – all aimed at improving antibody binding to specific immune cell receptors, thereby triggering a particular signaling pathway that either inhibits or stimulates the cell.
Targeting NKG2D to improve immunosurveillance
Among various immune cell populations, the receptors of natural killer (NK) cells and T lymphocytes (T cells) are important targets, as these cell types are one of the key players in tumor elimination. One of the stimulatory receptors that plays an important role in immunosurveillance of tumors and pathogens, is the activating receptor natural killer group 2 member D (NKG2D), which is expressed by NK cells, T cells and other immune cells.3 NKG2D or its ligand have been investigated as antibody-targets in cancer cell killing studies4–6 and the effect of NKG2D-immunotherapies in cancer patients has been evaluated in clinical trials with autologous T or NK cells bearing NKG2D chimeric antigen receptor (CAR).7
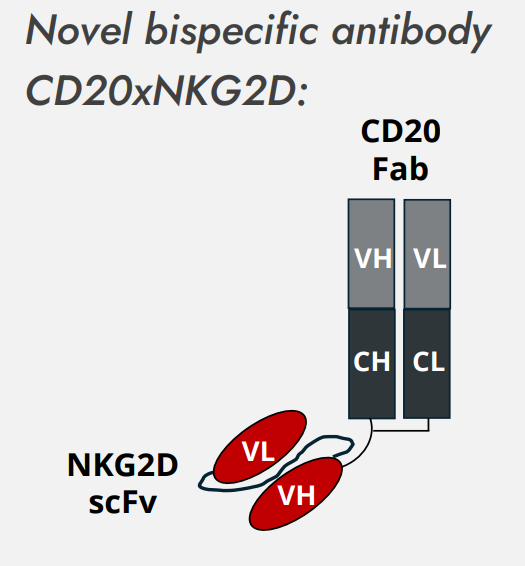
In a collaborative approach of the Universities of Kiel, Munich, and Braunschweig with YUMAB GmbH, novel NKG2D-specific antibodies have been developed to enhance lymphocyte recruitment and mediate killing of B cell lymphoma cells (GRANTA-519).8 Using phage display technology, a set of NKG2D-specific antibodies (scFv) was discovered and the most potent candidates were fused to an antigen-binding fragment (Fab) derived from the CD20-specific monoclonal antibody rituximab to generate a bsAb [CD20xNKG2D].
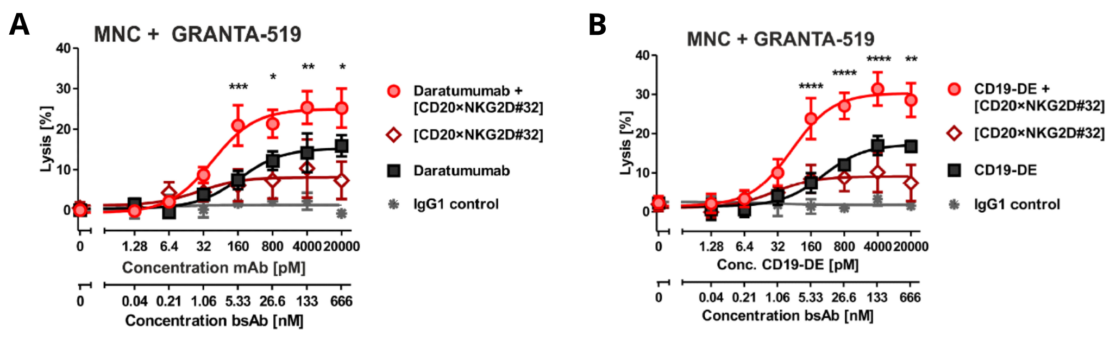
Lutz and coworkers demonstrated an in vitro activation of NK cells with this novel bsAb. Furthermore, the combination of [CD20xNKG2D] with the therapeutic antibody anti-CD38 (Daratumumab) or an Fc-engineered anti-CD19 antibody enhanced antibody-dependent cell-mediated cytotoxicity (ADCC) by NK cells, as shown in vitro in CD38+/CD20+ or CD19+/CD20+ expressing GRANTA-519 cells (Figure 1. A-B).
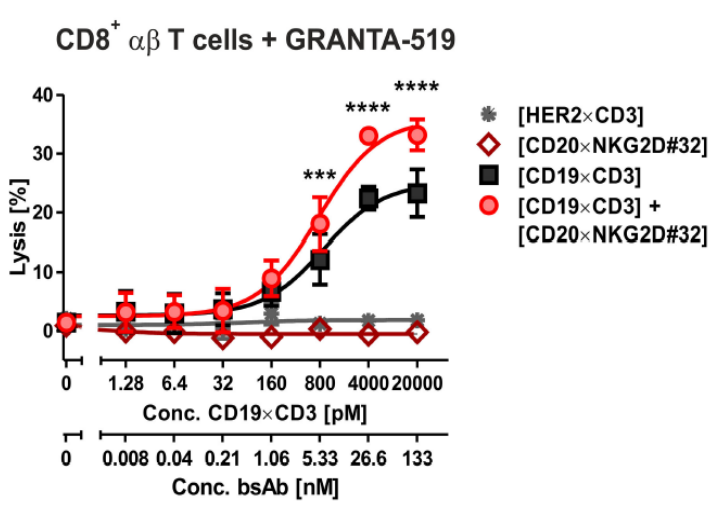
The combination of [CD20xNKG2D] and a T cell engager bsAB [CD19xCD3] in a dual-dual approach significantly increased CD8+ (αβ T cell receptor) T cell-mediated lysis of GRANTA-519 cells (Figure 2.).
CONCLUSION
Lutz and colleagues demonstrated that bispecific antibodies can enhance the efficacy of immunotherapy by including mAb or NK/T cell engager molecules in a combinatory approach.
The immune potential of the novel bispecific antibody needs be tested in animal models as a next step towards clinical development of a new cancer immunotherapy.