Small extracellular vesicles and particles (sEVPs) derived from tumor-free pre-metastatic organs promote breast cancer metastasis and support organotropismNew Collaborative Research Centre on Cancers of Blood-Forming Bone Marrow

Abstract
Metastatic breast cancer remains largely incurable, partly due to our incomplete understanding of its intricate underlying mechanisms. Notably, inercellular communication mediated by small extracellular vesicles and particles (sEVPs) has emerged as a key feature of metastasis. While tumor-derived sEVPs have been extensively studied and are known to be pro-metastatic, the role of sEVPs from metastasis-prone normal tissue sites remains primarily undefined. Here, we characterized and studied the function of sEVPs secreted from tumor-free pre-metastatic organs (TuFMO-sEVPs) such as the brain and lungs in both immunocompetent and patient-derived xenograft models. TuFMO-sEVPs from the brain of mammary tumor-bearing mice were found to have a distinct protein content as compared to brain-sEVPs from tumor-free mice, suggesting that the primary tumor can systemically influence the cargo of TuFMO-sEVPs. Importantly, mice orthotopically injected with breast cancer cells which had been educated with either brain or lung TuFMO-sEVPs prior to transplantation showed significantly increased metastasis to the respective organ. We further demonstrated that TuFMO-sEVPs induced the expression of the enzyme dihydrofolate reductase (DHFR) upon uptake by breast cancer cells, leading to their enhanced metastatic capacity. Organ-specific signatures generated from TuFMO-sEVP educated tumor cells were found to be increased in metastatic samples from breast cancer patients as compared to the primary tumor or normal tissue samples and these signatures also significantly correlated with poorer patient outcome. Collectively, our data reveals a novel facet of the metastatic cascade, implicating a role for TuFMO-sEVPs in directing metastasis and providing a potential therapeutic strategy for targeting this process.
Background
Metastasis is a dauntingly complex process which remains a major obstacle in the treatment of solid cancers including breast cancer. Metastatic breast cancer (MBC) arises when the primary tumor disseminates pioneer cells which travel through the blood or lymphatic system to distant organs such as the brain, lungs, bones and liver to form new colonies [1]. Notably, cell-cell communication is a key feature of this multi-stage process [2]. Besides local communication within the primary tumor microenvironment, cancer cells can also signal over long distances to sites of future metastases inducing the formation of a hospitable microenvironment termed as the pre-metastatic niche (PMN) which fosters the survival and outgrowth of disseminated tumor cells upon their arrival to these secondary sites [3, 4].
Importantly, extracellular vesicles and particles (EVPs) have emerged as key mediators of intercellular communication, both locally and systemically [2]. EVPs encompass a heterogeneous group of membrane-bound vesicles including small EVPs (sEVPs) which are usually less than 200 nm in diameter [5]. sEVPs are secreted by most, if not all, normal and cancer cells and enclose a multitude of bioactive molecules ranging from nucleic acids to proteins, metabolites and lipids. Once released from donor cells, sEVPs can transfer their functional cargoes to proximal and distant recipient cells, influencing their phenotype and function [6].
In cancer, sEVP-mediated bidirectional communication occurs between both malignant and non-malignant cells in the tumor microenvironment [2]. Specifically, tumor-derived sEVPs have been shown to play pleiotropic roles in reprogramming the host tissue and promoting cancer progression [6, 7]. They are also known to drive the formation of the pre-metastatic niche through enhanced vasculogenesis, extracellular matrix remodeling and immune regulation [3, 4, 8]. Besides tumor-derived sEVPs, the cellular components of the immediate tumor microenvironment namely epithelial cells, cancer-associated fibroblasts (CAFs), adipocytes, endothelial cells and immune cells also release sEVPs and support cancer progression by modulating tumor cell proliferation and metastasis [2, 6]. Of note, both tumor- and non-tumor-derived sEVPs may serve as novel biomarkers given that they are widely distributed in body fluids and contain specific contents which can be used to identify their cell of origin [9].
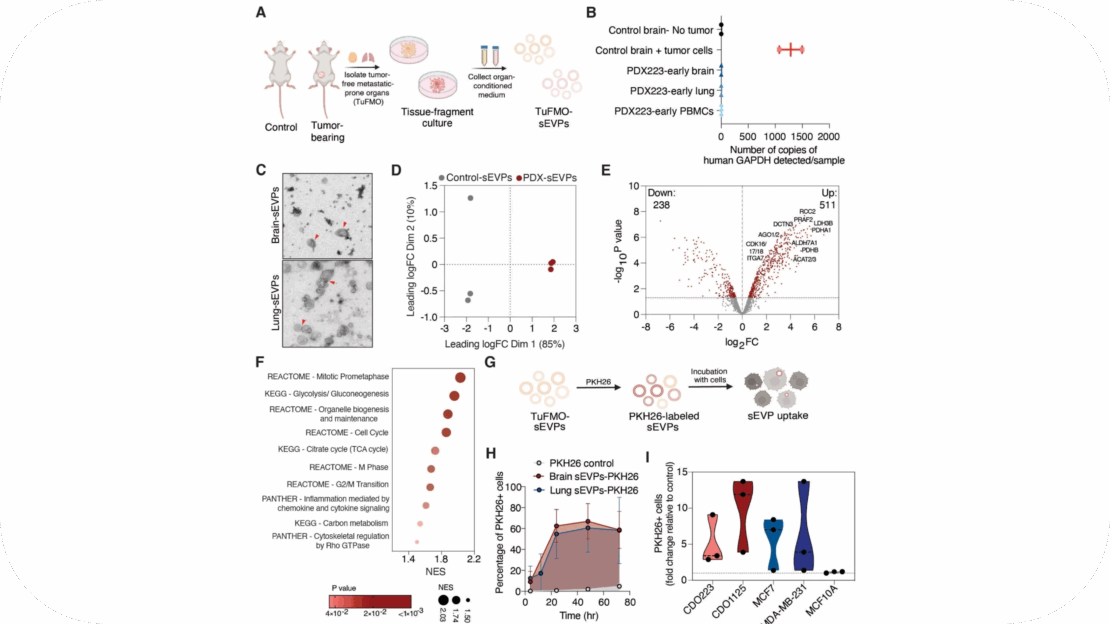
Importantly though, the function of sEVPs derived from normal tissues including tumor-free pre-metastatic sites such as the brain and lungs in breast cancer remains poorly characterized. While brain and lung-derived sEVPs are known to play key roles in both normal tissue homeostasis and brain- and lung-related disorders respectively [10], little is currently known about the effects of these sEVPs on tumor cells, especially in the context of tumor cell dissemination and outgrowth during breast cancer metastasis. This highlights the need to uncover the potential functions of tumor-free pre-metastatic organ-derived sEVPs (TuFMO-sEVPs) to better understand and treat metastatic disease.
In this study, we provide functional evidence for the role of brain and lung TuFMO-sEVPs in promoting breast cancer metastasis, with potential future clinical implications.
Conclusion
Our data collectively propounds that metastatic organs are not only passive recipients of tumor cells but that they can also secrete sEVPs which likely play a key role in the metastatic cascade. Given that tumor-derived sEVPs can travel to distant metastatic organs, it is plausible that TuFMO-sEVPs can conversely migrate to the primary tumor or interact with circulating tumor cells to promote the early stages of the metastatic cascade and metastatic organotropism. Future studies will be required to shed light on the physiological interactions between cancer cells and TuFMO-sEVPs from metastatic organs besides the brain and lungs and further explore the use of TuFMO-sEVPs as biomarkers in metastatic breast cancer patients. In conclusion, our study posits that the crosstalk of tumor cells with TuFMO-sEVPs helps in determining the cancer cell fate, providing a novel facet to our current understanding and management of metastatic organotropism and breast cancer metastasis.




