TRANSVAC: a pillar of vaccine development in Europe for 16 years

The European vaccine development ecosystem has been strengthened over the last 16 years, thanks to the EVI-led TRANSVAC project, an ambitious initiative funded by the European Commission. Since its launch in 2009, TRANSVAC has established itself as a key player in advancing vaccine research, supporting over 160 vaccine projects while fostering innovation and collaboration in the field.
A pioneering infrastructure for vaccine development
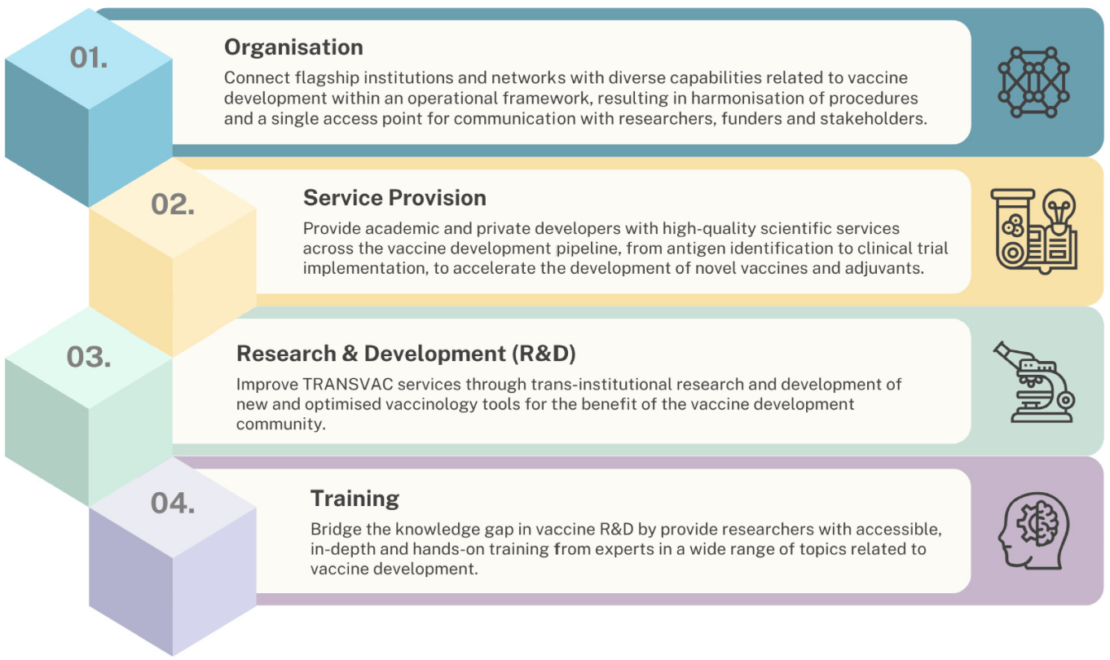
A recent article co-authored by experts from 11 leading institutions within the TRANSVAC network highlights its transformative role in supporting vaccine development over the past 16 years. TRANSVAC was designed to create a sustainable vaccine research infrastructure to meet the challenges of developing vaccines for a variety of diseases. The initiative has offered a broad range of services, including preclinical and clinical development support, technology transfer, and specialised training for researchers. By providing access to cutting-edge facilities and expertise, TRANSVAC has to date supported over 160 vaccine projects, aiming to accelerate the vaccine development pipeline by shortening the time between laboratory breakthroughs and clinical application.
Supporting researchers and industry
The TRANSVAC project has been instrumental in providing researchers and vaccine developers with access to guidance from experts and a broad array of state-of-the-art services that span preclinical and clinical development. Provided services range from antigen characterisation and production to preclinical immunology, vaccine formulation, and support for regulatory procedures and clinical trials. These services have enabled vaccine developers to address critical bottlenecks, ensuring the smooth progression of projects from concept to clinical trials both in academia, SMEs and industry.
Building expertise through training
In addition to providing technical services, TRANSVAC has prioritised capacity building. Through tailored training programs and workshops, the initiative has equipped researchers, clinicians, and technical staff with the skills needed to advance vaccine research and development. This focus on education aims to ensure that Europe remains at the forefront of vaccine innovation, with a highly skilled researchers ready to address emerging health threats. The program is continuing to grow and evolve to meet the changing needs of vaccine developers as the TRANSVAC Academy.
Preparing Europe for future health challenges
While celebrating the significant contributions of TRANSVAC, the article also emphasises the need to adapt to the evolving demands of vaccine research. It highlights key recommendations for future efforts, including enhanced flexibility in service offerings, increased support for pandemic preparedness, and strengthened partnerships between academia and industry. These steps are seen as essential to meet the challenges of emerging infectious diseases and to ensure timely responses to global health emergencies.
Looking ahead, the insights gained from TRANSVAC’s achievements offer a roadmap for strengthening vaccine infrastructure to address future health challenges. By fostering innovation, collaboration, and training, TRANSVAC is well positioned to lead in vaccine innovation and tackle both current and future health challenges.
For further details on TRANSVAC’s impact and achievements, read the full article here.