Common cough syrup ingredient shows promise in treating serious lung disease
EMBL scientists discover that an FDA-approved, over-the-counter cough syrup ingredient has potential to treat fibrotic lung disease.

Summary
- Lung fibrosis occurs when lung tissue becomes damaged, thickened, and scarred, leading to breathing difficulties that reduce the oxygen entering in the blood, which can be fatal. Lung fibrotic disease has several causes.
- According to research based on the 2019 Global Burden of Disease, fibrotic disease accounts for 17.8% of global deaths, and currently no cure is available.
- In collaboration with other researchers, EMBL scientists employed a variety of ‘omics’ technologies and microscopy to screen for a potential treatment for fibrosis, testing a library of FDA-approved drugs.
- Dextromethorphan proved to be most effective. It is FDA-approved as an active ingredient in cough syrups and cough/cold treatments, sold without a prescription around much of the world. This study shows that dextromethorphan has potential to also be applied as an anti-lung fibrosis drug in the future.
A common over-the-counter ingredient in many cough syrups may have a greater purpose for people suffering from lung fibrosis that is related to any number of serious health conditions.
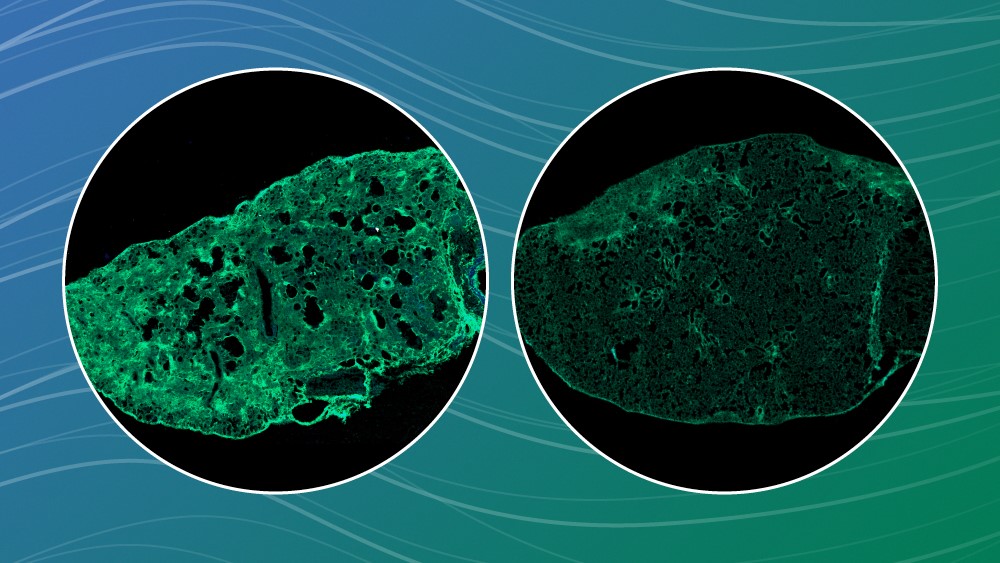
Scientists from EMBL Heidelberg were part of a collaborative effort to discover an effective treatment for lung fibrosis and found that the best candidate may be one that is already available as a cough medicine around the world, dextromethorphan. The study was recently published in Science Translational Medicine and showed how dextromethorphan can impede the collagen that forms scars inside cells, reducing lung fibrosis.
Lung fibrosis – caused by an excess amount of scarring, formed when fibrillar collagen accumulates in the lung – most often occurs in older adults for a variety of reasons: exposure to environmental irritants such as asbestos, coal dust, and mould; as a side effect from chemotherapy agents; as a long-term consequence from serious lung diseases such as tuberculosis; and as part of certain autoimmune or inflammatory diseases, such as lupus or rheumatoid arthritis.
Fibrotic scarring causes lung tissue stiffness, which leads to breathing difficulties and reduces oxygen concentration in the bloodstream, ultimately resulting in organ failure. Since 2019, the World Health Organization (WHO) estimates that 761,000 people in its European region suffer from lung fibrosis. Additionally, WHO estimates that 25,000 patients have died and 496,000 healthy years have been lost because of lung fibrosis.
“After learning that lung fibrosis has no cure available, I wanted to discover novel drugs for this condition,” said Muzamil Majid Khan, EMBL research associate and the paper’s first author.
Knowing the challenge of developing new drugs, Khan and the team decided to explore drugs that are already approved and readily available. EMBL scientists screened a library of FDA-approved drugs, including dextromethorphan. In collaboration with the Translational Lung Research Center (TLRC) Heidelberg and German Center for Lung Research (DZL), the researchers worked with human lung cells and applied a variety of cutting-edge technologies that didn’t even exist when many of these drugs were first approved.
“To screen for a potential anti-fibrotic drug, we used first, high-throughput microscopy of an optimised ‘scar-in-a-jar’ assay to identify potential drugs inhibiting collagen trafficking, followed by a variety of techniques such as proteomics, transcriptomics, microscopy. These allowed us to pin down the mechanisms of action of the drug,” Khan said. The ‘scar-in-a-jar’ assay gets its name from being an in vitro system to study lung fibrosis, allowing the scientists to address the complete process of collagen formation and thus use it as a model to test the efficacy of anti-fibrotic drugs.
These previous experiments led to testing the dextromethorphan in mouse models of lung fibrosis, but also in live 3D organotypic human lung tissue cultured in the lab.
“Being part of a consortium with DZL allowed us to collaborate with a local clinic in Heidelberg – Thoraxclinic – and we are now in the process of planning phase II clinical trials that can investigate if these same findings convey to what works in human patients,” said Rainer Pepperkok, EMBL group leader and senior author of this paper.
Together with further assistance from EMBL’s Proteomic Core facility, Chemcore, and medicinal chemists, the scientists plan to further investigate the drug, why it works and how it works. This will hopefully identify the target(s) the drug is working on in cells in the disease context, offering the possibility to develop improved variants of it.
“Investigating the trafficking of collagen was interesting on its own from a cell biological point of view, but it is now also potentially impactful from a disease point of view,” Pepperkok said. “It is important to remember that this is still fundamental research and only a very first step in understanding dextromethorphan’s impact on lung fibrosis. That said, this fundamental discovery does seem to offer promising therapeutic potential.”
Source article(s)
Dextromethorphan inhibits collagen and collagen-like cargo secretion to ameliorate lung fibrosis
Khan M.M., et al.
Science Translational Medicine 18 December 2024
10.1126/scitranslmed.adj3087




