Scientists discover the function of a mysterious HIV component
A research team including scientists from Heidelberg University Hospital has gained new insights into HIV-1.
Researchers from Martinsried, Heidelberg und Yale have discovered the mechanism behind an important step in the life cycle of HIV. Working together with teams at Heidelberg and Yale Universities, they found that the enigmatic “spacer peptide 2”, one of the virus components, plays a key role in converting immature HIV-1 particles into infectious particles. The results of the study were published in the journal Nature.
HIV-1 particles are released from infected cells in an immature, non-infectious form. The main building material for a virus particle is about 2000 copies of a long, rod-shaped protein called Gag. To become infectious, HIV has to undergo a maturation process. This involves the HIV-1 protease (a viral enzyme), that cuts up Gag into six smaller proteins including the capsid protein and the matrix protein. This causes a huge structural rearrangement of the virus components.
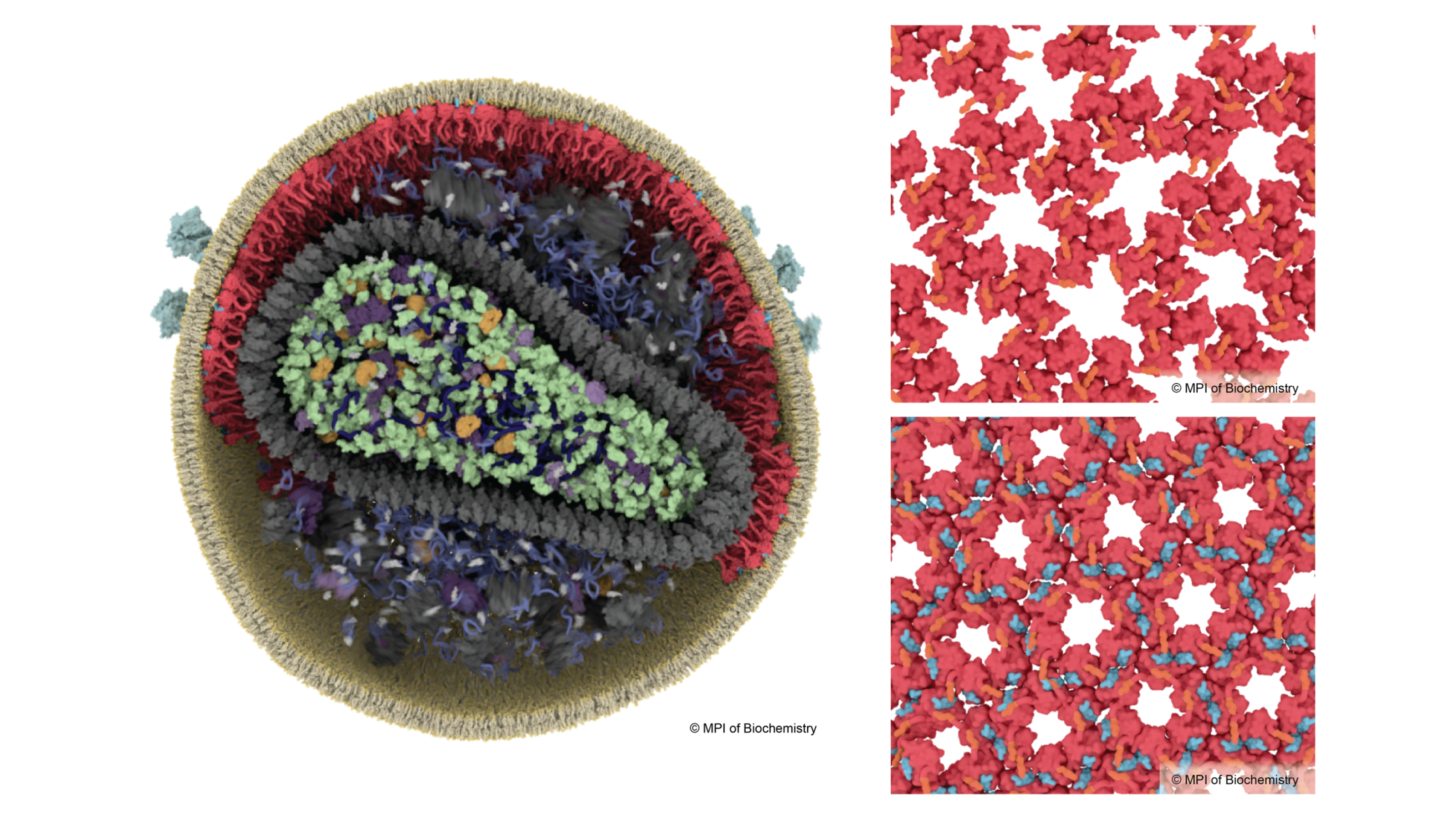
For many years, scientists have explored the structural changes of the virus capsid which encases the genome. In contrast, much less is known about the HIV-1 matrix – the outer protein shell directly beneath the lipid membrane that envelops the virus. Researchers led by John Briggs, Director and structural biologist at the Max Planck Institute of Biochemistry, have now worked out how the matrix protein rearranges during maturation into the infectious particle. They used the latest cryo-electron microscopes to image virus particles, and then used computational image analysis to derive very detailed 3D models of the viral proteins. Unexpectedly, they found that the rearrangement of matrix is triggered by “spacer peptide 2” which sticks to matrix and causes it to pack together in a different way. Spacer peptide 2 is another one of the six components formed by cutting up Gag, but its function was unknown until now. Binding of spacer peptide 2 to matrix protein allows the virus to fuse with target cells more quickly. The work was performed together with the research groups of Hans-Georg Kräusslich and Barbara Müller from the Department of Infectious Diseases, Virology, of Heidelberg University and partners from Yale University, USA.
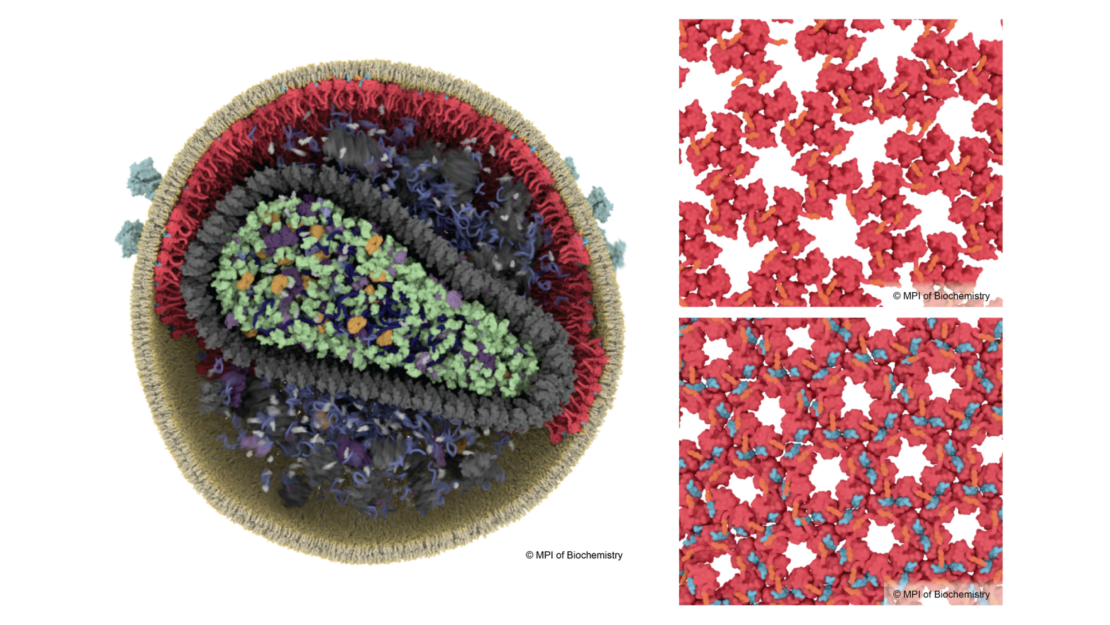
John Briggs explains: “In our lab, we obtained the first structural data about the matrix in 2021, but we didn’t know what caused it to rearrange when the virus matures. In this new study we produced much more detailed 3D views of the matrix layer, that was key to understanding what is going on.”
James Stacey and Dominik Hrebik the two first authors of the study explain their findings. James says: “The virus matrix has a pocket in its mature form. We knew that something binds there, but we thought it was a lipid from the membrane. Now we can see that it is spacer peptide 2. We wonder whether this pocket could be a target for drug molecules in the future” Dominik says: “Until now, the function of spacer peptide 2 was not known. Thanks to high-resolution cryo-electron microscopy, we have seen that this peptide, after its release, binds directly to the matrix proteins and links the proteins together in the mature virus.”
John Briggs summarizes: “HIV-1 is probably the most studied virus, but there are still important steps in its replication we don’t yet understand.”
Original Publication
James C. V. Stacey, Dominik Hrebík, Elizabeth Nand, Snehith Dyavari Shetty, Kun Qu, Marius Boicu, Maria Anders-Össwein, Pradeep D. Uchil, Robert A. Dick, Walther Mothes, Hans-Georg Kräusslich, Barbara Müller & John A. G. Briggs: The conserved HIV-1 spacer peptide 2 triggers matrix lattice maturation, Nature, February 2025
DOI: 10.1038/s41586-025-08624-9
https://www.nature.com/articles/s41586-025-08624-9