Quality standards for looking into the tumor genome
Personalized medicine with individually tailored therapies is becoming more and more a reality in cancer. This requires a precise look into the genetic material of tumors, a molecular diagnostic tumor profile. A research group from the German Network for Personalized Medicine (DNPM) has recorded the quality standards according to which genome analyses are carried out in Germany. The data is a prerequisite for integrating gene sequencing into routine care. The working group led by Professor Albrecht Stenzinger from the Medical Faculty of Heidelberg University was in charge of the project.

Hardly any other medical field is changing as rapidly as cancer medicine. This applies to knowledge about tumor diseases as well as the development of new therapies. In recent decades, research has shifted more and more to the molecular biological level. Increasingly, the genetic characteristics of tumors are being deciphered, some of which are suitable targets for targeted therapies. The vision for the future is to create a molecular biological profile for each individual tumor, which can be used as a basis for the therapy concept.
First a letter salad, then a book
It is now possible to “read out” individual genes, a selection of genes or even the entire genetic material of a person. Gene sequencing, panel sequencing, exome and genome sequencing are the technical terms. The genetic material – DNA (deoxyribonucleic acid) – is encrypted information that uses only a few letters. Molecular pathologists such as Prof. Dr. Albrecht Stenzinger, head of the study and head of the Molecular Pathology Center at the Pathology Institute of the UKHD, are able to read and understand this code better and better. To do this, the tumor sample is first processed, broken down into its building blocks together with its genetic information and then analyzed. The resulting “letter salad” is then organized and structured by bioinformaticians with the help of high-performance computers. The result is a readable book containing the genetic information of the respective patient, or more precisely, their tumor.
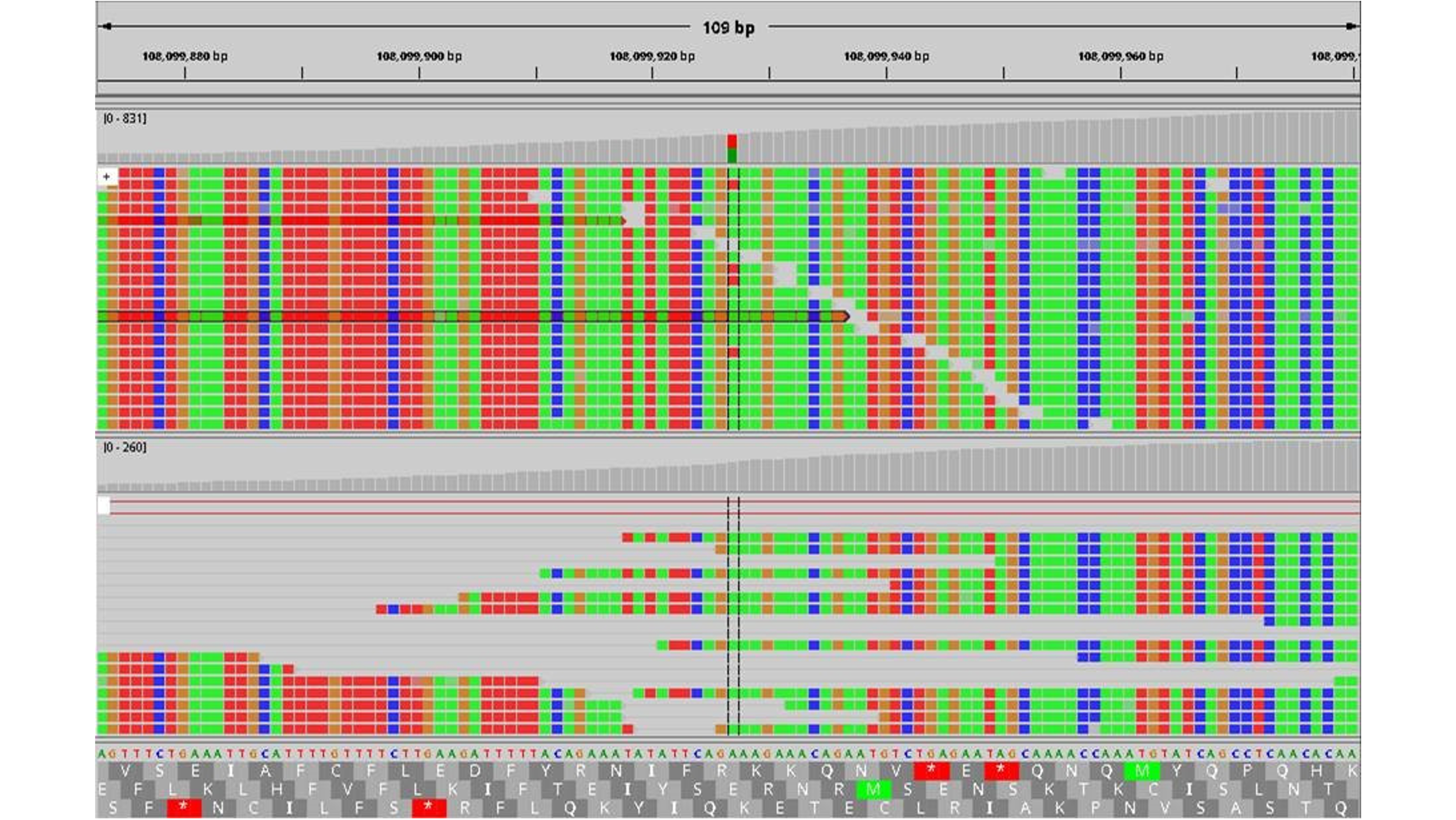
A quality-assured procedure is essential here. This is the only way to guarantee that the sequencing of one and the same sample in two different institutes leads to an identical result and therefore also to the appropriate therapy. However, which method is used to sequence the genetic material and how good the result of the analysis is has not yet been systematically recorded in Germany. At least not as far as the sequencing of a particularly relevant part of the genome, the so-called exon, is concerned. Exons are all sections of genetic information that contain blueprints (codes) for proteins. The entire genome is much more extensive.
Germany-wide test run
For their inventory, the researchers therefore had six tumor samples and four already known reference samples examined at a total of 21 university cancer centers spread across Germany. “The vast majority of all sequence analyses are carried out at university hospitals. This gave us a good overview of the quality of tumor sequencing in Germany,” says Professor Stenzinger.
The test results showed a high level of agreement between the results from different institutes, but not complete agreement. The next step for the research group is therefore to develop standards that can be used to optimize the quality of exon sequencing. One starting point is the filtering of the numerous genetic deviations found in individual tumor samples. They must be checked for relevance with the help of large databases. “Most deviations are of no further significance, but some drive tumor growth or give the cancer cells particular resistance and are therefore rewarding therapeutic targets. If we standardize the filter criteria more, we can increase the agreement of the test results to almost 100 percent,” says Dr. Michael Menzel, first author of the article and bioinformatician at the Institute of Pathology at the UKHD.
Personalized medicine is teamwork
The pilot project `genomDE´ initiated by the German Federal Ministry of Health has recently been launched with the aim of anchoring comprehensive sequencing in standard care on a trial basis. At the same time, data is to be collected in this way that will benefit future cancer research. This is known as knowledge-generating care. The costs of gene sequencing will be funded by the health insurance companies. After five years, it will be reviewed whether the benefits justify the costs. If so, gene sequencing is to be integrated into standard care in the long term.
However, quality-assured personalized medicine is only possible in an interdisciplinary team with a wide range of expertise, as Prof. Stenzinger emphasizes. The Heidelberg working group is part of the nationwide Network for Personalized Medicine (DNPM) with a number of certified centers that are intended to be pioneers and successfully work together on an interdisciplinary basis.
Literature
Menzel M, Martis-Thiele M, Goldschmid H, et al. Benchmarking whole exome sequencing in the German network for personalized medicine. Eur J Cancer. Published online September 8, 2024. https://doi.org/10.1016/j.ejca.2024.114306